Keywords
Abstract
Purpose: The purpose of the present study was to quantify test-retest reproducibility of measurements of stiffness of the human trabecular meshwork (HTM) by atomic force microscopy (AFM).
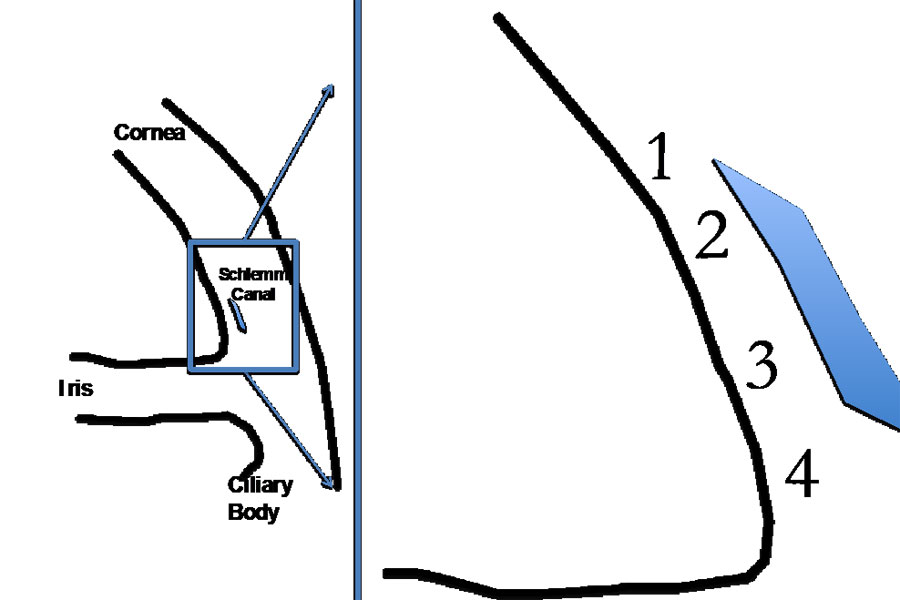
Methods: Eleven 40 μm radial limbal cryostat sections from a fresh human donor rim were mounted on charged slides and rehydrated at room temperature. Stiffness at four TM locations (anterior to posterior along Schlemm’s canal) was measured by AFM. At each location, a 6 x 6 grid was sampled. Indentation points were evenly distributed over a 20 μm x 20 μm area, with a rate of one load/unload cycle per second. Measurements were then repeated for calculation of test-retest variability.
Results: The test-retest coefficients of variation for the four measurement locations (anterior to posterior) were 24.39, 25.28, 12.74, and 14.26%, respectively, with a notable drop in the two posterior locations compared to the anterior. The test-retest coefficient for the sections was 19.17%. For the entire eye, the test-retest coefficient of variation for the measurement of the TM stiffness was 17.13%. Young’s moduli consistently decreased from anterior to posterior location.
Conclusions: Wide regional variation suggests that single value does little to fully describe the complex array of TM stiffness levels within the eye, and future studies of TM stiffness assessed by AFM should include multiple tissue samples from each eye, with documentation of the anterior-posterior location of each measurement.
References
Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821-829.
Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661-1669.
Sommer A, Tielsch JM. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1996;114:235.
Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study G. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699-708.
Leske MC, Hyman L, Hussein M, Heijl A, Bengtsson B. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1999;127:625-626.
American Academy of Ophthalmology, Glaucoma Panel, Preferred Practice Pattern Guidelines. Primary Open-Angle Glaucoma. San Francisco CA, AAO. 2010.
Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147-2152.
Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Invest Ophthalmol Vis Sci. 2012;53:5242-5250.
Yu JG, Bao FJ, Feng YF, et al. Assessment of corneal biomechanical behavior under posterior and anterior pressure. J Refract Surg. 2013;29:64-70.
Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55:814-823.
Huang J, Camras LJ, Yuan F. Mechanical analysis of rat trabecular meshwork. Soft Matter. 2015;11(14):2857-2865.
Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget. 2015.
Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. Wnt inhibition induces persistent increases in intrinsic stiffness of human trabecular meshwork cells. Exp Eye Res. 2015;132:174-178.
Candiello J, Balasubramani M, Schreiber EM, et al. Biomechanical properties of native basement membranes. FEBS J. 2007;274:2897-2908.
Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29:402-410.
Ruozi B, Tosi G, Forni F, Fresta M, Vandelli MA. Atomic force microscopy and photon correlation spectroscopy: two techniques for rapid characterization of liposomes. Eur J Pharm Sci. 2005;25:81-89.
Trache A, Meininger GA. Atomic force-multi-optical imaging integrated microscope for monitoring molecular dynamics in live cells. J Biomed Optics. 2005;10:064023.
Tocha E, Schonherr H, Vancso GJ. Quantitative nanotribology by AFM: a novel universal calibration platform. Langmuir. 2006;22:2340-2350.
Tsuji T, Kobari K, Ide S, Yamanaka K. Suppression of spurious vibration of cantilever in atomic force microscopy by enhancement of bending rigidity of cantilever chip substrate. Rev Sci Instrum. 2007;78:103703.
Ying ZC, Reitsma MG, Gates RS. Direct measurement of cantilever spring constants and correction for cantilever irregularities using an instrumented indenter. Rev Sci Instrum. 2007;78:063708.
Akrami SM, Nakayachi H, Watanabe-Nakayama T, Asakawa H, Fukuma T. Significant improvements in stability and reproducibility of atomic-scale atomic force microscopy in liquid. Nanotechnology. 2014;25:455701.
Demichelis A, Divieto C, Mortati L, Pavarelli S, Sassi G, Sassi MP. Toward the realization of reproducible Atomic Force Microscopy measurements of elastic modulus in biological samples. J Biomech. 2015;48:1099-1104.
Braunsmann C, Hammer CM, Rheinlaender J, Kruse FE, Schaffer TE, Schlotzer-Schrehardt U. Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Invest Ophthalmol Vis Sci. 2012;53:2960-2967.
Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A. 2013;110:6370-6375.
Radmacher M, Fritz M, Hansma PK. Imaging soft samples with the atomic force microscope: gelatin in water and propanol. Biophys J. 1995;69:264-270.
Freddo T, Johnson M. Aqueous Humor Dynamics I: Measurement methods and animal studies. In: Civan MM (ed), The Eye’s Aqueous Humor. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier; 2008.
Harris AR, Charras GT. Experimental validation of atomic force microscopy-based cell elasticity measurements. Nanotechnology. 2011;22:345102.
Vargas-Pinto R, Gong H, Vahabikashi A, Johnson M. The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys J. 2013;105:300-309.